Hello!
The answer is: 500g
Why?
We are asked to find the theoretical yield of a reaction, and we are given the following information:
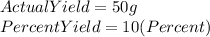
In order to find the the theoretical yield we must use the following formula:
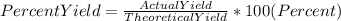
We have to convert the percents to real numbers before the calculations. We can do it dividing the percent value into 100, so:
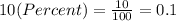
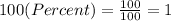
Therefore,
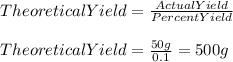
Have a nice day!