Hello!
The answer is: 4.77atm
Why?
Since there's a fixed volume, we can use the the Gay-Lussac's Law which stablish a relation between the pressure and the temperature:
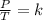
P is the volume of the gas
t is the temperature of the gas
k is the proportionality constant
We also have the following equation:
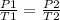
Where:
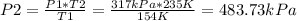
We are asked to find the pressure in atm, so we must convert 483.73kPa to atm:
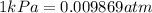
Then,
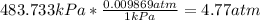
Have a nice day!