Answer:The correct answer is ;
The oxidation state of nitrogen in NO changes from +2 to 0, and the oxidation state of carbon in CO changes from +2 to +4 as the reaction proceeds.
Step-by-step explanation:
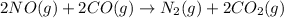
In an oxidation recation addition of oxygen atom takes place or loss of electrons takes place.
In an reduction reaction removal of oxygen atom takes place or gain of electrons takes place.
In the given reaction , the nitrogen atom is present in +2 oxidation state in NO molecule and present in 0 oxidation state in
 molecule. Hence, nitrogen is getting reduced that is reduction reaction. NO is oxidizing agent
molecule. Hence, nitrogen is getting reduced that is reduction reaction. NO is oxidizing agent
In the given reaction , the carbon atom is present in +2 oxidation state in CO molecule and present in +4 oxidation state in
 molecule. Hence ,carbon is getting oxidized that is oxidation reaction. CO is a reducing agent.
molecule. Hence ,carbon is getting oxidized that is oxidation reaction. CO is a reducing agent.