Hello!
The answer is: -97.23° C
Why?
Applying the Charles and Gay-Lussac's Law we have that:
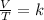
Where:
k is the proportionality constant
v is the volume of the gas
t is the temperature of the gas
We also have the following relation:
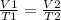
Since we need to know the new temperature (T2) we can find it using the last equation, so:
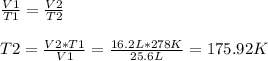
We are asked to find the temperature in Celsius degrees, we must convert the result:
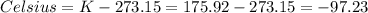
Therefore, the temperature is -97.23° C
Have a nice day!