Answer:

Step-by-step explanation:
We are given the chemical formula:
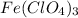
There are 3 elements here:
- Fe: Iron
- Cl: Chlorine
- O: Oxygen
The question asks for the number of oxygen atoms, so we can just focus on the O in the formula.
The O has a subscript of 4, indicating there are 4 oxygen atoms in the compound. But the compound is also enclosed in parentheses with a subscript of 3. Therefore, there are 3 of the compounds with 4 oxygen atoms.
We can multiply 3 and 4.
There are 12 oxygen atoms.