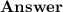
Aluminum ions have a charge of +3. This means that the atoms have three more protons than electrons. In order to add electrons to aluminum ions, the purification process therefore requires a large amount of electricity.
Uncharged Aluminum atom must need to lose it's electrons,in order to form the bond with oxygen which has vacant orbitals
ion
atom that has a positive or negative charge because it lost or gained one or more electrons
chemical bond
the attractive force that holds atoms or ions together
ionic bond
a chemical bond in which one atom loses an electron and the other atom gains electrons to form ions
chemical formula
a combination of chemical symbols and numbers to represent a substance
covalent bond
bond formed by the sharing of electrons between atoms
Thanks