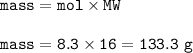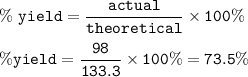The percent yield : 73.5%
Further explanation
Given
Reaction
C+2H₂⇒CH₄
Required
The percent yield
Solution
mol of Carbon(as a limiting reactant) :
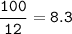
mol CH₄ based on C, and from equation mol ratio C : CH₄, so mol CH₄ = 8.3
Mass of Methane(theoretical yield) :