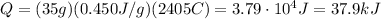Answer:
37.9 kJ
Step-by-step explanation:
We can calculate the thermal energy gained by the iron using the formula:
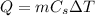
where
m = 35 g is the mass of the iron
Cs = 0.450 j/g is the iron's specific heat capacity
 is the change in temperature of the iron (assuming that the room's temperature is 20 C degrees)
is the change in temperature of the iron (assuming that the room's temperature is 20 C degrees)
Substituting numbers into the formula, we find