The molecular formula : Al₂Cl₆
Further explanation
Given
20.2% by mass of Aluminum and 79.8% by mass of Chlorine.
Required
the molecular formula
Solution
Aluminium (Ar=27 g/mol)
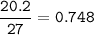
Chlorine(Ar=35.5 g/mol)
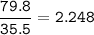
mol ratio Al : Cl =
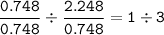
So the empirical formula = AlCl₃
(Empirical formula)n=Molecular formula
(27+3.35.5)n=267
(133.5)n=267⇒n=2
The molecular formula : Al₂Cl₆