Answer: The correct answer is Option D.
Step-by-step explanation:
The reaction of HCl with every substance given in the option follows the equations:
The reaction of aluminium with hydrochloric acid produces aluminium chloride and hydrogen gas. The equation follows:
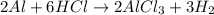
Thus, this substance evolve gas on reaction.
The reaction of zinc with hydrochloric acid produces zinc chloride and hydrogen gas. The equation follows:
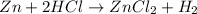
Thus, this substance evolve gas on reaction.
- Option C:
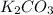
The reaction of potassium carbonate with hydrochloric acid produces potassium chloride, water and carbon dioxide gas. The equation follows:
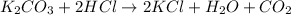
Thus, this substance evolve gas on reaction.
- Option D:
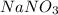
The reaction of sodium nitrate with hydrochloric acid produces sodium chloride and nitric acid. The equation follows:
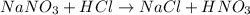
Thus, this substance will not evolve gas on reaction.
Hence, the correct answer is Option D.