1.) Find The Number of Half Lives Passed:
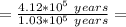 half lives
half lives
2.) Calculate Number of Ca Atoms remaining:
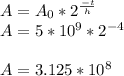 atoms left
atoms left
3. Calculate Number of Potassium Atoms
Subtract the final number of Calcium atoms from the initial amount of calcium atoms.
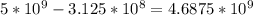 Atoms of Potassium
Atoms of Potassium