The balanced chemical equation for the production of chromium metal from the reaction of chromium(ll) nitrate reacts with a strip of zinc is:
3 Zn + 2 Cr(NO₃)₃ → 2 Cr + 3 Zn(NO₃)₂
This is a redox reaction, which is a chemical reaction in which one or more electrons are transferred between the reagents, causing a change in their oxidation states. In the proposed reaction, Cr oxidation state goes from +3 to 0, becoming metallic chromium, while Zn goes from being Zn⁰ to Zn²⁺.
The mass of chromium metal produced in the above reaction will be,
425.0 mL x
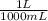 x
x
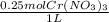 x
x
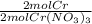 x
x
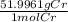 = 5.52 g
= 5.52 g
So, the mass of chromium metal produced when 425.0mL of 0.25M chromium(ll) nitrate reacts with a strip of zinc that remains in excess is 5.52 g of Cr.