Answer: b. 12.9 grams
Explanation:
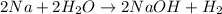
According to avogadro's law, 1 mole of every gas occupies 22.4 Liters at STP ,contains avogadro's number
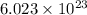 of particles and weighs equal to the molecular mass.
of particles and weighs equal to the molecular mass.
Thus at STP :
1 mole of hydrogen is produced by 2 moles of sodium metal
i.e. 22.4 L of
 is produced by 46 gram of sodium metal
is produced by 46 gram of sodium metal
6.3 L of
 is produced by=
is produced by=
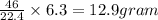 of sodium metal
of sodium metal