Answer: Option (A) is the correct answer.
Step-by-step explanation:
Specific heat is defined as the amount of heat needed to raise the temperature by one degree celsius.
Mathematically, Q =
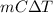
where Q = heat absorbed
m = mass
C = specific heat
 = change in temperature
= change in temperature
Therefore, in 1 kg there are 100 grams so, 10 grams equal 0.01 Kg. Thus, calculate the specific heat value as follows.
Q =
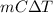
100 joules =
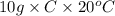
C = 0.5
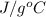
Thus, we can conclude that specific heat of the given metal is 0.5
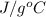 .
.