- Symbol of the ion: Br⁻.
- Name of the ion: Bromide ion.
Step-by-step explanation
Br is the symbol for the element bromine. It is found in new IUPAC group 17 of a modern periodic table. Bromine is a halogen.
To name a halogen anion with charge -1, replace the suffix "-ine" in the name of the element with the suffix "-ide." For example,
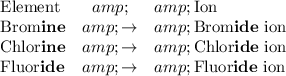
The symbol of a bromine atom is Br. The atom is neutral. However, it carries a negative charge of -1 after it gains one electrons and forms a negative ion. The charge of the bromide ion shall be written as a superscript in its symbol. The size of the charge is written in front of the sign.
The charge on the bromide ion is -1. In the superscript, omit the number 1 and write "-". Hence the symbol
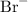 .
.