Step-by-step explanation:
Helium is the element of 18th group and first period. The electronic configuration of helium is - 2 or
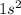
There are 2 valence electrons of helium.
Only the valence electrons are shown by dots in the Lewis structure.
As, stated above, there are only two valence electrons of helium, so in the Lewis structure, two dots are made around the helium symbol.
Neon is the element of 18th group and second period. The electronic configuration of neon is - 2, 8 or
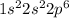
There are 8 valence electrons of neon.
Only the valence electrons are shown by dots in the Lewis structure.
As, stated above, there are only two valence electrons of neon, so in the Lewis structure, eight dots are made around the neon symbol.
Hence, the electron dot structures will be different for both of them.