Step-by-step explanation:
When we write a chemical equation then the number present in front of each element or compound is known as the coefficient.
For example,
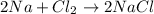
Here, 2 present before Na represents that amount of sodium is twice the amount of chlorine which leads to the formation of 2 moles of sodium chloride salt.
Therefore, we can conclude that to double the amount of sodium, you need to add the coefficient of 2.