First, you need to find the mass of 1 mol of sugar. Mass, or molar mass, can simply be found by adding the masses of the individual elements. These are given to you on the periodic table.
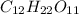
12 x 12.011 grams (molar mass of Carbon) = 144.132 g
22 x 1.008 grams (molar mass of Hydrogen) = 22.176 g
11 x 15.999 grams (molar mass of Oxygen) = 175.989 g
Add all of the pieces together.
144.132 g + 22.176 g + 175.989 g = 342.297 grams
So, if one mole has 342.297 grams, then 7.35 of that amount will be your answer.
342.297 g/mol x 7.35 mol = 2,515.88 grams