39.2 g/mol.
Step-by-step explanation
The molar mass of a substance is the same as the mass of one mole of the substance.
Under Standard Temperature and Pressure, STP, the volume of one mole of an ideal gas is 22.4 liters. As a result, under STP the mass of one mole of this gas will be the same as the mass of 22.4 L of the same gas.
The density of this gas is 1.75 g/L. The mass of each liter of the gas is 1.75 grams. The mass of 22.4 L of the same gas will thus equal
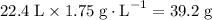 .
.
The mass of one mole or 22.4 L of this gas is 39.2 g. As a result, the molar mass of this gas is
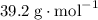 .
.
To generalize,
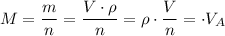 ,
,
where
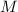 is the molar mass of the gas;
is the molar mass of the gas;
 is the density of the gas; and
is the density of the gas; and
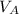 is the volume of one mole of the gas,
is the volume of one mole of the gas,
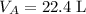 under STP.
under STP.