The concentration of the solution is also known as Molarity (M). The formula for molarity is
M = mol / liters
We already have the liters, so we only need to find moles. We can find the number of moles by using the molar mass of NaCl and the given amount. That can be found using the elements' atomic weights given on the periodic table.
22.990 (mass of Na) + 35.453 (mass of Chlorine) = 58.443 g / mol
That's how many grams are in one mole of NaCl, but we only have 2.5 grams. So, let's divide and see how many moles we have.
2.5 / 58.443 = .042776 moles
Now we can plug our numbers into the formula.
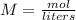
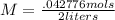
M = .02138 mols/L
So, your concentration is about .02 mol/L.