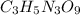Empirical formulas are the lowest whole number ratio of compounds. The first step to finding them is to imagine you have 100g of the unknown compound. Treat each percent as a weight, since 2.22% of 100 is 2.22, you can say you have 2.22 grams of Hydrogen in 100 g of your unknown. The next thing you want to do is divide each mass by the molar mass of the element you're working on (found on the periodic table). Then divide all of those numbers by the lowest one you found.
Carbon:
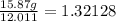
Hydrogen:
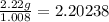
Nitrogen:
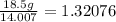
Oxygen:
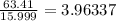
The lowest number is 1.32076, so divide all of them by that.
Carbon:
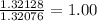
Hydrogen:
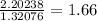
Nitrogen:
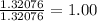
Oxygen:
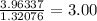
Ideally, all of these should be whole numbers. However, 1.66 is not close enough to be rounded to 2. To fix this, we need to multiply every number by a whole number that will make them all close to whole numbers. Since we only have one weird decimal, let's just find out what will make it a good, whole number.
1.66 x 2 = 3.32
1.66 x 3 = 4.98
4.98 is close enough to round to 5, so let's multiply the rest of our numbers by 3 as well.
Carbon: 1 x 3 = 3
Hydrogen: 1.66 x 3 ≈ 5
Nitrogen: 1 x 3 = 3
Oxygen: 3 x 3 = 9
These are the numbers for your final empirical formula. You can make sure it's empirical by making sure the numbers can't be simplified by a common factor.