Answer: The frequency is directly proportional to energy of the wave.
Step-by-step explanation:
Frequency is defined as the number of waves that passes a given point per second. It is expressed in Hertz. It is denoted by the symbol

The equation that links energy of the particle and frequency of the particle is given by Planck's equation, which is:
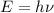
where,
E = energy of the particle
h = Planck's constant
 = frequency of the particle
= frequency of the particle
Hence, the frequency is directly proportional to energy of the wave.