Hello!
The answer is: C2H6
Why?
First, we need to find the empirical formula of the compound:
Looking for the relative atomic mass for each element:
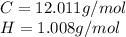
Finding the number of moles for each element:
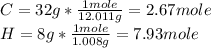
Then, we need to determinate the mole ratio by dividing each result into the smallest number:
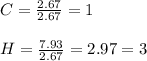
So, the empirical formula would be CH3
We are given the molar mass of the compound is equal to 30 g/mol
We need to compare the empirical formula molecular mass and the molecular formula molar mass in order to find the number of atoms of the compound:
Empirical formula molar mass:
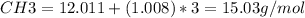
Molecular formula molar mass = 30.0 g/mol
So, by dividing the molecular formula molar mass by the empirical formula molar mass we have the number of atoms of the compound:
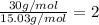
Therefore,
The molecular formula will be: (CH3)2= C2H6
Have a nice day!