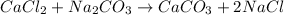Answer:
The products in the following double displacement reaction is
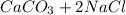
Step-by-step explanation:
Ina double displacement reaction, cations and anions of two ionic species exchange their partners, forming new compounds.
Here (
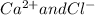 ) and (
) and (
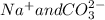 ) are two different ionic pairs. Reaction between these two pairs resulting exchange of ionic species between them. Hence
) are two different ionic pairs. Reaction between these two pairs resulting exchange of ionic species between them. Hence
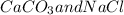 are formed as products of the given reaction.
are formed as products of the given reaction.