Answer:
No, the amount of matter of the reactants is not equal to the amount of matter of the products.
Step-by-step explanation:
According to the conservation of matter the amount of matter of the reactants is equal to the amount of matter of the products. The given reaction is
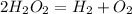
In the reactant side there are
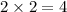 molecules of hydrogen and
molecules of hydrogen and
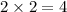 molecules of oxygen.
molecules of oxygen.
In the product side there are 2 molecules of both hydgrogen and oxygen. So the reaction does not satisfy the law of conservation of mass.
The balanced reaction would be
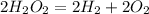 .
.