Answer:
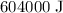
Step-by-step explanation:
m = Mass of ice = 200 g
 = Temperature change of water =
= Temperature change of water =

c = Specific heat capacity of water = 4200 J/kg °C
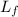 = Specific latent heat of fusion = 340 kJ/kg
= Specific latent heat of fusion = 340 kJ/kg
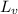 = Specific latent heat of vaporisation = 2260 kJ/kg
= Specific latent heat of vaporisation = 2260 kJ/kg
Heat required to convert ice to water =
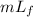
Heat required to raise the temperature of water to boiling point =
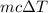
Heat required to convert water to steam =
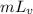
Total heat required
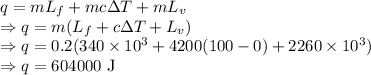
Heat required to convert the given amount of ice to steam at the required temperature is
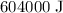 .
.