Hello!
The answer is:
1 - Find the chemical formula of the compound.
2 - Find the relative atomic mass of each element using the periodic table: Finding the relative atomic mass of each element of the compound will help us to find the compound molecular mass. Relative atomic mass tells us how many grams are in each mole of that element.
3 - Find how many atoms of each element are in the compound.
4 - Multiply each relative atomic mass by the number of atoms in order to know the Molar Mass of each element.
5 - Sum each molar mass element of the compound in order to find the total molecular mass: Remember that the Molar Mass of the compound is the sum of each element molecular mass in the compound.
For example, let's find the Molar Mass of the Glucose:
1 - Glucose chemical formula:
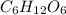
2 - Finding the relative atomic mass of each element:
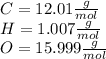
3 - Finding how many atoms/molecules of each element we have:
C = 6 atoms
H = 12 atoms
O = 6 atoms
4 - Multiplying each relative atomic mass by the number of atoms in order to get the Molar Mass of the elements:
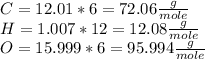
5 - Finding the Molar Mass of the compound by the sum of each Molar Mass:
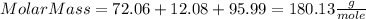
Have a nice day!