Answer: A) 0.5 J / g ºC
Step-by-step explanation:
The specific heat capacity or specific heat is a physical quantity that is defined as the amount of heat that must be supplied to the mass unit of a thermodynamic substance or system to raise its temperature by one unit.
So, the specific heat capacity (c) will be given by,
c = q / m ΔT
where q is the transfer of energy between the substance and its environment or another substance, m is its mass of and ΔT is the temperature increase experienced by the system.
If q = 100 J, m = 10 g and ΔT = 20°C, then
c =
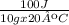 → c = 0.5 J / g ºC
→ c = 0.5 J / g ºC
So, the specific heat capacity of a metal if 100 joules are needed to raise 10 g of water by 20°C is c = 0.5 J / g ºC