Oxidation state of sulfur S: +5.
Step-by-step explanation
Known oxidation states:
- Na: +1. Sodium Na is a main group metal. The oxidation state of Na in compounds should be positive and the same as its group number. The new IUPAC group number (1 ~ 16) of Na is 1. The oxidation state of Na should thus be +1.
- O: -2. Oxygen is the second most electronegative element on the periodic table. The oxidation state of oxygen is mostly -2 with a few exceptions:
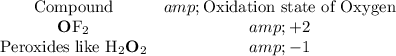 .
.
What the question is asking for:
- The oxidation state of S can vary from compound to compound. Let the oxidation state of S in
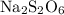 be
be
 .
.
The oxidation state of each atom in a compound should add up to zero.
- There are two Na atoms in this formula. The oxidation state on each is +1. Add
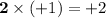 ;
; - There are six O atoms in this formula. The oxidation state on each is -2. Add
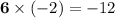 ;
; - There are two S atoms in this formula. The oxidation state of each is assumed to be
 . Add
. Add
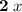 .
.
Sum of oxidation states on each atom in
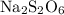 :
:
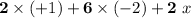 .
.
Again, this value should equals to zero since
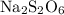 is overall a neutral compound.
is overall a neutral compound.
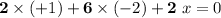 ;
;
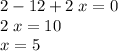 .
.
Thus the oxidation state of sulfur in
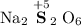 should be +5.
should be +5.