Answer:
The volume that 3 moles of gas particles occupy is 67.2 L (option D)
Step-by-step explanation:
The STP conditions refer to the standard temperature and pressure. Pressure values at 1 atmosphere and temperature at 0 ° C are used and are reference values for gases. And in these conditions 1 mole of any gas occupies an approximate volume of 22.4 liters.
Then it is possible to apply a rule of three as follows: if according to STP 1 mole is available in 22.4 L, 3 moles in how much volume is it?
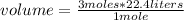
volume=67.2 L
The volume that 3 moles of gas particles occupy is 67.2 L (option D)