Answer:
The final volume of the gas is 0.752 L.
Step-by-step explanation:
Initial pressure of the gas =
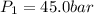
Initial volume of the gas =
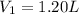
Initial temperature of the gas =
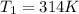
Final pressure of the gas =
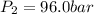
Final volume of the gas =
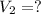
Final temperature of the gas =
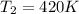
Number of moles of gases are constant
Using an ideal gas equation :

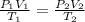
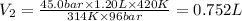
The final volume of the gas is 0.752 L.