Answer: Oil: covalent
Cornstarch: Covalent
Sodium chloride: Ionic
Sodium bicarbonate: Ionic
Explanation: Covalent compounds are formed by sharing of electrons between non metals whereas ionic compounds are formed by transfer of electrons from metals to non metals.
1. Oil, which is built from the nonmetals hydrogen, carbon, and oxygen: forms a covalent compound by sharing of electrons between non metals hydrogen, carbon, and oxygen. Covalent compounds are insoluble in water.
2. Cornstarch, a carbohydrate consisting of hydrogen, carbon, and oxygen: forms a covalent compound by sharing of electrons between non metals hydrogen, carbon, and oxygen. Covalent compounds are insoluble in water.
3. Sodium chloride (table salt), whose formula is NaCl is formed by transfer of electrons from sodium to chlorine.Ionic compounds are soluble in water.
4. Sodium bicarbonate, whose formula is
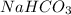 is formed by transfer of electrons from sodium to
is formed by transfer of electrons from sodium to
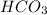 .Ionic compounds are soluble in water.
.Ionic compounds are soluble in water.