Answer:
Work done: 0 J
Change in internal energy: +100 J
Step-by-step explanation:
- The work done by the gas is given by:
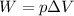
where
p is the gas pressure
 is the change in volume of the gas
is the change in volume of the gas
Since the gas is kept in a rigid container, its volume is fixed, so there is no change in volume: therefore,
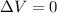 and the work done is zero as well:
and the work done is zero as well:
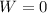
- The change in internal energy of the gas is given by the 1st law of thermodynamics:
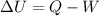
where Q is the heat supplied to the gas. Since W=0 and Q=+100 J, then the change in internal energy of the gas is
