Answer:
Position and momentum
Step-by-step explanation:
According to Heisenberg uncertainty principle, it is not possible to find the position and momentum of an electron simultaneously. Mathematically, it can be written as :
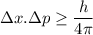
Where
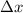 is uncertainty in position
is uncertainty in position
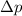 is uncertainty in momentum
is uncertainty in momentum
The above equation shows that if we know the position of an electron accurately, then its momentum cannot be known accurately and vice versa.
Hence, the correct option is (b) "Position and momentum".