Answer:
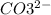 +
+
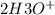 =>
=>
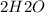 +
+
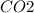
Step-by-step explanation:
Nitric acid reacts with sodium carbonate to form sodium nitrate, carbon dioxide and water.
Sodium carbonate + nitric acid => sodium nitrate + water + carbon dioxide
2HNO3 + Na2O3 => 2NaNO3 + CO2 + H2O
So the net ionic equation is as follows:
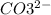 +
+
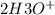 =>
=>
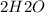 +
+
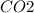
The sodium ion and the nitrate ions are not involved in the net equation because they are not changed in the reaction.