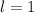 .
.
Step-by-step explanation
Each electron in an atom comes with a unique set of four quantum numbers.
 gives the main shell of an electron.
gives the main shell of an electron.
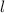 gives the type of the sub-level (a.k.a. orbital, s, p, d, f, ...) that the electron occupies.
gives the type of the sub-level (a.k.a. orbital, s, p, d, f, ...) that the electron occupies.- There can be more than one orbitals in a sub-level. Consider
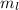 as the index for the electron's orbital.
as the index for the electron's orbital. - Each orbital holds two electrons.
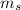 tells those two electrons apart based on the direction of each electron's spin.
tells those two electrons apart based on the direction of each electron's spin.
The question is asking about different types of sub-levels. That depends on the angular / azimuthal quantum number,
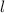 .
.
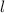 is an integer. It ranges from
is an integer. It ranges from
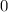 all the way to
all the way to
 .
.
- An electron with
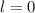 is in an
is in an
 orbital.
orbital. - An electron with
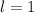 is in a
is in a
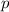 orbital.
orbital. - An electron with
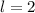 is in a
is in a
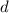 orbital.
orbital.
In other words, the quantum number
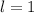 represents a
represents a
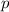 sublevel.
sublevel.
Reference
Vitz, Ed et al. "Quantum Numbers (Electronic)", ChemPRIME (Moore et al.). Chemistry Libretexts.
"Quantum Mechanics and Atomic Orbitals", Chemistry - The Central Science (Brown et al.). Chemistry Libretexts.