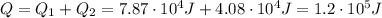Answer:
Step-by-step explanation:
1) First of all, we need to calculate the heat needed to raise the temperature of the block of lead from the initial temperature (25.0 C) to the melting temperature (327.5 C). This heat is given by the equation
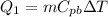
where
m = 2.00 kg is the mass of the block
Cpb = 130.0 J/kg*C is the specific heat capacity of lead
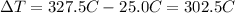 is the temperature difference
is the temperature difference
Substituting,
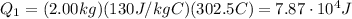
2) Now we have to calculate the amount of heat needed to completely melt the lead, which is given by:
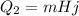
where
Hj = 2.04x10^4 J/kg is the latent heat of fusion of lead
Substituting,
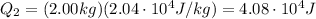
3) Therefore, the total heat needed is