Answer:
For a. Neutrons and electrons also form an atom.
For b. The element is oxygen which is a non-metal and will form a negative ion while forming ionic bond.
Step-by-step explanation:
There are 3 subatomic particles which form an atom. These are neutrons, protons and electrons.
Neutrons carry no charge, protons carry positive charge and electrons carry negative charge. Neutrons and protons are present in nucleus and electrons revolve around the nucleus.
The energy which is present between neutrons and protons are nuclear energy and the energy which is present between electrons and protons are electrostatic energy.
In an element, number of protons is always equal to the number of electrons. The atomic number is equal to the number of protons or electrons. The element which has atomic number 8 is Oxygen.
The electronic configuration of this element is
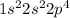
This element requires only 2 electrons to form a stable electronic configuration. An element which gains electron is considered as a non-metal and forms a negative ion because number of electrons increases.