Answer: Temperature of the substance determines the average kinetic energy of particles in a gas.
Step-by-step explanation:
Average kinetic energy is defined as the average of all the kinetic energies of the particles in a system. It is a measure of temperature of the particles in a gas.
The equation used to calculate the average kinetic energy of the particles is given as:
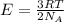
where,
R = Gas constant
T = Temperature of the gas
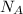 = Avogadro's number
= Avogadro's number
E = Average kinetic energy of the particles
As, temperature is directly related to the average kinetic energy of the gas. Thus, the temperature of the particles increases, the average kinetic energy of the particles also increases and vice-versa.
Thus, temperature of the substance determines the average kinetic energy of particles in a gas.