Phosphorus pentachloride.
Step-by-step explanation
There are two elements in PCl₅:
- P- Phosphorus;
- Cl- Chlorine.
Both P and Cl are nonmetals. Nonmetals tend to share electrons to form covalent molecules. PCl₅ is likely to be a covalent molecule. The name of PCl₅ and other molecules of two elements comes in a couple of parts:
- First prefix- How many atoms of the first element (P in this case) in the molecule? Di- for two, tri- for three, etc. There's no mono- here. Omit the first prefix if there's only one atom of the first element in the molecule (as in this case.)
- Name of the first element. Phosphorus.
- Second prefix- How many atoms of the second element (Cl in this case) in the molecule? Similar to the first prefix, however include "mono-" if there's only one atom of the second element in the molecule. There are five Cl atoms in each molecule. Hence the prefix "penta-."
- Name of the second element as if it was an anion. The anion from Cl is the chloride ion Cl⁻. Hence the name "chloride."
Combining the parts:
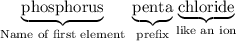 .
.
Hence the name: phosphorus pentachloride.