Answer 45 : This statement is true.
Explanation :
We are taking an example of carbon (C) with atomic number 6 and atomic mass 12.
As we know that the number of protons and number of electrons are equal to the atomic number.
The atomic number of carbon is 6. So, the number of protons and the number of electrons is equal to 6.
And, the number of neutrons = Atomic mass - Atomic number = 12 - 6 = 6
Hence, this statement is true.
Answer 46 : This statement is true.
Explanation :
We are taking an example of oxygen (O) with atomic number 8.
The electronic configuration of oxygen (O) will be,
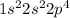
Now we have to calculate the number of electrons from the electronic configuration.
The number of electrons = 2 + 2 + 4 = 8
Hence, this statement is true.