Answer:
pH at equivalence point will be 7
Step-by-step explanation:
 is a strong acid and KOH is a strong base. Hence a neutral salt would appear from acid-base reaction between them.
is a strong acid and KOH is a strong base. Hence a neutral salt would appear from acid-base reaction between them.- Acid-base reaction:
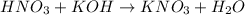
- At equivalence point,
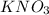 and water exists in medium.
and water exists in medium. - As
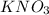 is a neutral salt therefore pH of medium will be equal to 7 (neutral).
is a neutral salt therefore pH of medium will be equal to 7 (neutral). - So, pH at equivalence point will be 7.