Answer: The given statement is false.
Step-by-step explanation:
Acids are the substances that release hydrogen or hydronium ions when dissolved in water.
For example,
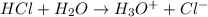
Whereas bases are the substances that release hydroxide ions when dissolved in water.
For example,
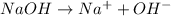
Therefore, we can conclude that the statement, concentration of an acid or base refers to the presence of H3O+ ions in solution, is false.