PART A)
One half life means the times after which half of the radioactive substance will decay. So here we have 8 g of isotope present in the sample
Half life of the sample = 6 minutes
Now we need to find the amount after 12 minutes
So it will complete the cycle of two half life
So it will decay to half two times in given time
So final amount will be m = 2 gram
PART B)
After every half life of the cycle the original amount will be reduced to half of initial
So here if the proportion of carbon-14 is 1/8 times of initial
So we can say it is
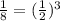
so it will complete half life 3 times
so it is 5730 yrs + 5730 yrs + 5730 yrs
so total life is T = 17190 years