Answer:
a. Mass number is the sum of number of protons and neutrons present in the nucleus of an atom.
A = p + n
Charge is a unit of matter that tells how more or less the number of electrons are present as compared to number of protons in an atom. A positive charge means the atom lacks electrons.
Gamma rays are highly energetic electromagnetic rays. They have high penetrating power and are very difficult to shield owing to high energy.
b. The compete nuclear equation is:
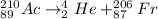
This is a alpha decay process because alpha particle which is Helium atom is being produced. Francium is being formed after alpha particle is emitted.
c. The complete nuclear reaction is:
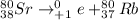
This is a positron emission process where in Rubidium is being formed.