Answer : The concentrations of hydroxide and hydronium ions in a solution with a pH of 10.2 are,
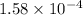 and
and
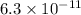 respectively.
respectively.
Explanation : Given,
pH = 10.2
pH : It is defined as the negative logarithm of the hydrogen ion concentration.
First we have to calculate the hydrogen ion concentration
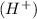
![pH=-\log [H^+]](https://img.qammunity.org/2020/formulas/chemistry/high-school/ipfjz05f4cfbguiwup37xvxa7furlbuapf.png)
Now put the value of pH in this formula, we get the hydrogen ion concentration.
![10.2=-\log [H^+]](https://img.qammunity.org/2020/formulas/chemistry/high-school/t8m5eg41xq98pay212br8li3qw7dos4xer.png)
![[H^+]=6.3* 10^(-11)](https://img.qammunity.org/2020/formulas/chemistry/high-school/kk8b8ndgbxq6yajytuo0k6b7ap98fitt2s.png)
Now we have to calculate the pOH of the solution.
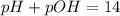
Now put the value of pH, we get the value of pOH.
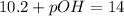
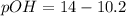
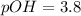
Now we have to calculate the hydroxide ion concentration
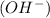
![pOH=-\log [OH^-]](https://img.qammunity.org/2020/formulas/chemistry/high-school/h1t4ubcsdqvqg0xpalkkvnwrun04y9pzd8.png)
Now put the value of pOH in this formula, we get the hydroxide ion concentration.
![3.8=-\log [OH^-]](https://img.qammunity.org/2020/formulas/chemistry/high-school/gati0oelo3ot2maq931tynbeq6vr21zqea.png)
![[OH^-]=1.58* 10^(-4)](https://img.qammunity.org/2020/formulas/chemistry/high-school/p2yumhbcgjbxfxbwrgt7o4t9tp6ibpf00t.png)
Therefore, the concentrations of hydroxide and hydronium ions in a solution with a pH of 10.2 are,
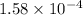 and
and
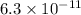 respectively.
respectively.