PART A)
efficiency of the heat engine is defined as
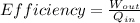
So it is ratio of output work and input Heat energy
So here we can say that
In order to increase the efficiency we have to increase the output work with less amount of input heat
this is related to temperature as
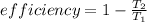
now we can say that in order to increase efficiency T2 must have to decrease and T1 have to increase
Part b)
now we can say that in order to increase efficiency T2 must have to decrease and T1 have to increase
So on decreasing the temperature T2 and increasing the temperature T1 we will get more efficiency of heat engine
Part c)
heat require to increase the temperature
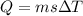
now we have
m = 16.1 kg
s = 4186 J/kg C
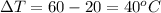
now we have
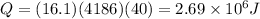
Part d)
Heat taken by heater = 6040 kJ
efficiency = 67%
now we have
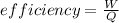
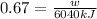
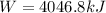
Part e)
Energy lost to the surrounding = Heat given - Work done
Energy lost = 6040 kJ - 4046.8 kJ
Energy lost = 1993.2 kJ