PART A)
In isotopes number of protons will be same in two.
So there is no difference in number of protons
PART B)
Number of neutrons will be different in two isotopes
it may be greater or less than by one
So two isotopes are of different number of neutrons
PART C)
Atomic number of atom is written in subscript of an atom
It is written below the atom
It shows the total number of protons or electrons in an atom
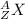 in this presentation z = number of protons
in this presentation z = number of protons
PART D)
iron-55 shows that it has number of protons + neutron = 55
As we know that atomic number of iron is 26
so number of protons = 26
now number of neutrons = 55 - 26 = 29
number of electrons = number of protons = 26