0.064387 mol of H₂O molecules were lost in this process.
There are 0.032439 mol of CaCl₂ in that sample.
Formula of the hydrate: CaCl₂ · 2 H₂O.
Step-by-step explanation
Relative atomic mass:
- H: 1.0078;
- O: 16.000;
- Cl: 35.456
- Ca: 40.078.
What's the mass of water lost in this process?
Assuming that water is the only source of mass lost in this process.
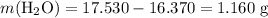 .
.
How many moles of molecules in that 1.160 grams of water?
Molar mass of water:
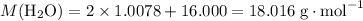 .
.
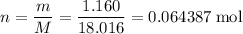 .
.
What's the mass of the salt?
There hasn't been any significant change in mass after the first heating. All water should have been removed. The anhydrous salt should account for the rest of the mass in the crucible.
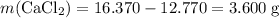 .
.
How many moles of formula units in that 3.600 grams of CaCl₂?
Molar mass of CaCl₂:
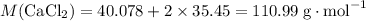 .
.
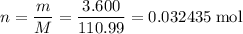 .
.
How many moles of water molecules in each mole formula unit of CaCl₂?
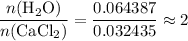 .
.
In other words, there are two moles of water molecules for every mole of CaCl₂ formula unit. Hence the formula: CaCl₂ · 2 H₂O.