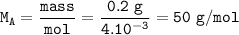The relative molecular mass of acid A : 50 g/mol
Further explanation
Given
40.0 cm³(40 ml) of 0.2M sodium hydroxide
0.2g of a dibasic acid
Required
the relative molecular mass of acid A
Solution
Titration formula
M₁V₁n₁=M₂V₂n₂
n=acid/base valence(number of H⁺/OH⁻)
NaOH ⇒ n = 1
Dibasic acid = diprotic acid (such as H₂SO₄)⇒ n = 2
mol = M x V
Input the value in the formula :(1 = NaOH, 2=dibasic acid)
0.2 x 40 x 1 = M₂ x V₂ x 2
M₂ x V₂ = 4 mlmol = 4.10⁻³ mol ⇒ mol of Acid A
The relative molecular mass of acid A (M) :